gas analysis based on the molar volume|1 mole of gas volume : maker The volume (\ (V\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (P) and the number of temperature (T) are constant. We can express this . Thanks for the info but i've got 3 questions 1- why do you hate fitgirl 😂😂 2- what are original scene releases 3- why should i block that image ? (Just curious) Ps: i stumbled upon that website, i didn't intentionally visit it Reply reply
{plog:ftitle_list}
Portal Triângulo TV, Araguari. 51,709 likes · 864 talking about this · 12 were here. Canal de TV em Araguari
In this experiment we shall measure the mass and corresponding volume of a sample of oxygen gas and from this data calculate the volume at standard conditions, then we shall calculate the volume of 32.0 g (1 mole) of oxygen gas.Molar volume is defined as the volume occupied by one mole of a gas. Using the ideal gas law and assuming standard pressure and temperature (STP), the volume of one mole of gas can .The volume (\ (V\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (P) and the number of temperature (T) are constant. We can express this .The Molar Volume of a Gas. Imagine you have a bottle of fizzy drink. We know that when we open a bottle, it fizzes a lot. Do you know that this fizz actually indicated that gas in leaving the .
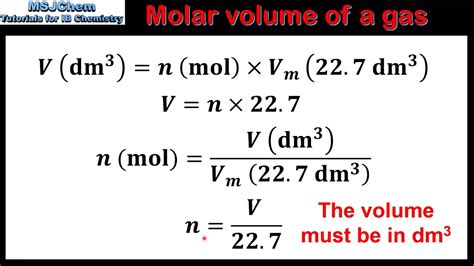
The molar volume of a gas is the volume of one mole of a gas at STP. At STP, one mole (\( 6.02 \times 10^{23}\) representative particles) of any gas occupies a volume of .Core Practical 1: Measuring the Molar Volume of a Gas. Measuring gas volumes. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water. Gas syringe equipment for .To put everything together as you analyze your data to confirm Avogadro’s hypothesis, you will use the Combined Ideal Gas Law to calculate the volume, at STP, of the hydrogen gas that .
Calculate the molar volume of a gas at STP using experimental data. Calculate the molar mass of a metal using experimental data. Introduction. Metals that are above hydrogen in the activity .If a gas is composed of different types of molecules (i.e., nitrogen, oxygen, etc.), the volume percentage of each gas is equal to the molar percentage of that gas. This can be shown as .Molar Volume of a Gas Lab. Teacher was Jubrail Rahil. Course. General Chemistry (202-NYA-05) 396Documents. Students shared 396 documents in this course. University. Dawson College. Academic year:2019/2020. Uploaded by: .
molar volume at normal conditions
The ideal gas equation can be rearranged to give an expression for the molar volume of an ideal gas: = = Hence, for a given temperature and pressure, the molar volume is the same for all ideal gases and is based on the gas constant: R = 8.314 462 618 153 24 m 3 ⋅Pa⋅K −1 ⋅mol −1, or about 8.205 736 608 095 96 × 10 −5 m 3 ⋅atm⋅K .Analysis. Read off the volume of gas produced for a sensible mass of sodium carbonate, e.g. 0.35 g produces 79.0 cm 3 . The mass of sodium carbonate may be specified in an exam questionAs before, we can use Avogadro’s law to predict what will happen to the volume of a sample of gas as we change the number of moles. Because \(V/n\) is a constant for any given sample of gas (at constant \(P\) and \(T\)), we can again imagine two states; an initial state with a certain number of moles and volume (\(V_1/n_1\)), and a final state with values for a different number .
(1) You can use the ideal gas equation, PV = nRT, to find the volume of 1 mole of ideal gas (molar volume of gas) at 100 kPa and other temperatures. (2) Prior to 1982, standard temperature and pressure were defined as 0°C (273.15 K) and 1 atm (101.3 kPa), so 1 mole of gas would occupy a volume of 22.41 LChemists sometimes make comparisons against a standard temperature and pressure (STP) for reporting properties of gases: 273.15 K and 1 atm (101.325 kPa). 1 At STP, one mole of an ideal gas has a volume of about 22.4 L—this is referred to as the standard molar volume ().
thickness measurement equipment
Capillary Columns. A capillary, or open tubular column is constructed from fused silica and is coated with a protective polymer coating. Columns range from 15–100 m in length with an internal diameter of approximately 150–300 μm. Figure 12.4.3 shows an example of a typical capillary column.It can be calculated by dividing the molar mass (M) by mass density (ρ). Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. Molar Volume Formula. The Molar volume is directly proportional to molar mass and inversely proportional to density. The formula of the molar volume is expressed as Experiment 2: Analysis of a KClO3 Mixture and the Molar Volume of Oxygen Prepared by: . Conclusion: • Based on the experimental result, the molar volume of the gas collected is L/mol. The molar volume of any gas at STP ("Standard temperature and pressure" of 273.15 K and 1 atm) is 22.414 L which gives a -3.91% difference between them. .Thus the volume of 1 mol of an ideal gas is 22.71 L at STP and 22.41 L at 0°C and 1 atm, approximately equivalent to the volume of three basketballs. The molar volumes of several real gases at 0°C and 1 atm are given in Table 10.3, which shows that the deviations from ideal gas behavior are quite small.
Asked for: volume of nitrogen gas produced. Strategy: A Calculate the number of moles of N 2 gas produced. From the data in Table 6.6.1, determine the partial pressure of N 2 gas in the flask. B Use the ideal gas law to find the volume of N 2 gas produced. Solution:Pre-Lab cleler nate re molar vdune aps tow corealuct xk che co yeac bon mad produces nae acleg to wl! coal que hi walabo measute ofs produ chon ob. achemic ac. Skip to document. . Molar Volume of a Gas Pre-Lab. Pre-Lab. Course. Experimental Chemistry I (CHEM 111) 145 Documents. Students shared 145 documents in this course. University With increasing molar mass, this method becomes increasingly inaccurate because of the concurrent decreasing concentration of the end groups so that simple titration methods can only be used for end group analysis of molar mass up to a maximum of ca. 20,000 g/mol. Spectroscopic methods such as UV-, IR-, or 1 H-NMR-measurements are also .
In other words, 1 mole of a gas will occupy 22.4 L at STP, assuming ideal gas behavior. At STP, the volume of a gas is only dependent on number of moles of that gas and is independent of molar mass. With this information we can calculate the density (\( \rho \)) of a gas using only its molar mass. First, starting with the definition of density
The molar volume is defined by following formula: (V= molar volume, v =volume, n= moles) V= v/ mol. The molar volume is being compared it to an ideal gas at standard conditions (0°C, 101 kPa). Molar volume is calculated through the following formula where V is molar volume, R is universal gas constant, T is temperature (K) and P is pressure . The quantity 22.41 L is called the standard molar volume The volume of 1 mol of an ideal gas at STP (0°C and 1 atm pressure), which is 22.41 L. of an ideal gas. The molar volumes of several real gases at STP are given in Table 6.3.1, which shows that the deviations from ideal gas behavior are quite small. Thus the ideal gas law does a good job .How to find the molar volume of a gas using the ideal gas law? The most common molar volume is the molar volume of an ideal gas at standard temperature and pressure (273 K and 1.00 atm). The molar volume is the . The old definition was based on a standard pressure of 1 atm. . The volume of 1 mol of an ideal gas at STP is 22.41 L, the standard molar volume. All of the empirical gas relationships are special cases of the ideal gas law in which .
This experiment uses two gas laws to determine the molar mass of butane: the ideal gas law and Dalton’s law of partial pressures. Gas samples are described using four variables: Pressure (P), Volume (V), moles (n), and Temperature (T). The ideal gas law combines these 4 .Based on the Ideal Gas Law, one can derive a very important relationship. If a gas is composed of different types of molecules (i.e., nitrogen, oxygen, etc.), the volume percentage of each gas is equal to the molar percentage of that gas. This can be shown as follows: Let V be the total gas volume and N be the total number of moles. The Ideal .
The Molar Volume of a Gas. From Avogadro's Law, the volume is directly proportional to the number of moles. The important point to keep in mind is that n can be moles of any gas, since one mole always contain 6.02 ×10 23 molecules, and one molecule of any gas at the same temperature will have the same kinetic energy.. Based on this principle it is found that one .Conversions Between Moles and Gas Volume. Molar volume at STP can be used to convert from moles to gas volume and from gas volume to moles. The equality of \(1 \: \text{mol} = 22.4 \: \text{L}\) is the basis for the conversion factor.
In addition to analyzing the unknown mixture for percent calcium carbonate, the _____ volume of carbon dioxide is also determined. At STP, one mole of an ideal gas occupies _____ L; that is, its molar volume is 22.4 L at STP. Because carbon dioxide is not an ideal gas, we may expect its molar volume to ..
the molar volume of an ideal gas is: 22.41 L/mol, i.e., exactly one mole of any ideal gas at 273.15 K and 101.3 kPa pressure will occupy a volume of 22.41 L. This volume is called the . molar volume. In this experiment, you will confirm that the molar volume of hydrogen gas at STP is indeed 22.41 L/mol.
The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions.. The most common example is the molar volume of a gas at STP (Standard Temperature and Pressure), which is equal to 22.4 L for 1 mole of any ideal gas at a temperature equal to 273.15 K and a pressure equal to 1.00 atm. .Avogadro's Law. Avogadro’s Law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of space; At room temperature and pressure, the volume occupied by one mole of any gas was found to be 24 dm 3 or 24,000 cm 3 This is known as the molar gas volume at RTP RTP stands for “room temperature and pressure” .- Gas Density: Molar volume is used to calculate the density of gases. By dividing the molar mass of a gas by its molar volume, chemists can determine the density of the gas in grams per liter (g/L). This information is valuable in various applications, such as determining the buoyancy of a gas or analyzing gas mixtures. .
molar gas volume meaning
Analysis. Read off the volume of gas produced for a sensible mass of sodium carbonate, e.g. 0.35 g produces 79.0 cm 3 . The mass of sodium carbonate may be specified in an exam question
thickness measurement units
corneal thickness measurement
WEBOlá, Aqui Disponibilizamos Um Quiz Sobre Aqueles Conceitos Básicos De Anatomia Humana. Treine Para Arrasar Nas Provas! Bons Estudos!! <3 nosso Instagram: @Marias_odonto
gas analysis based on the molar volume|1 mole of gas volume